Pipeline
I Development Pipeline
EnnoDC has a growing pipeline of novel immunotherapies for cancers such as HPV16+ oropharyngeal and prostate cancer, as well as infectious diseases including COVID and HIV.
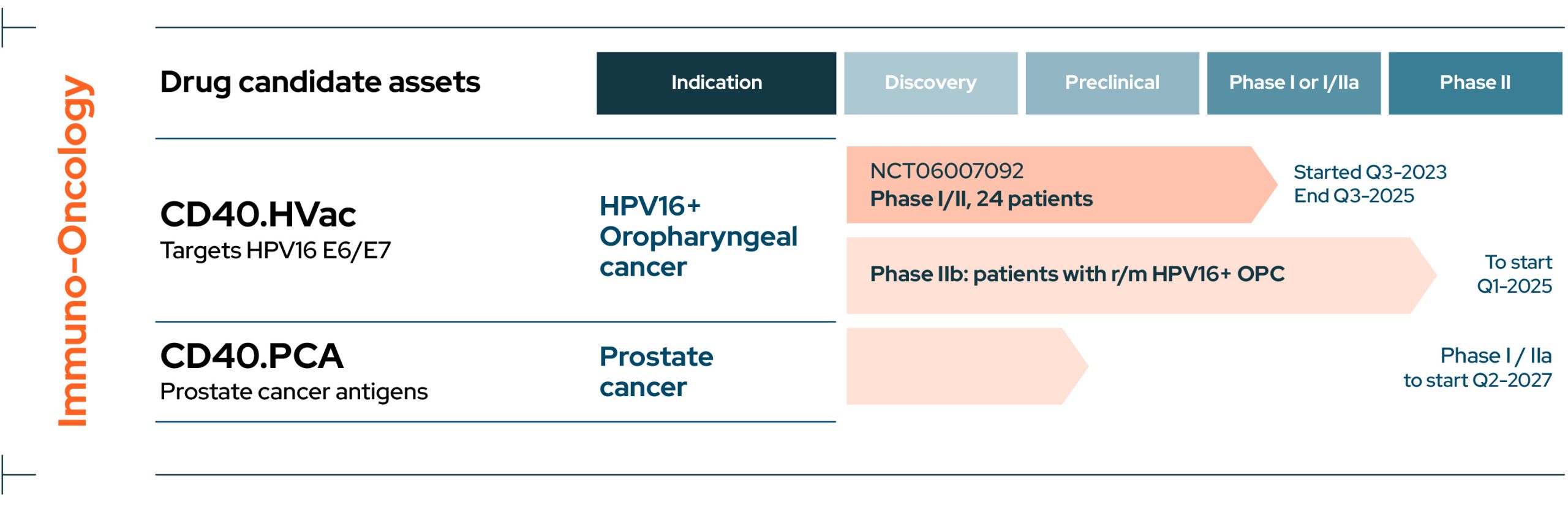
Cancer
View Drug Candidate Assets
CD40.HVac
CD40.HVac is an immunotherapy targeting the human papilloma virus HPV16 E6/E7 antigen in patients with HPV16+ oropharyngeal carcinoma and is currently in an ongoing Phase I/II multicentric double-blind placebo-controlled dose escalation trial (NCT06007092).
The study is being conducted in collaboration with the Gustave Roussy Cancer Campus.
CD40.PCA
CD40.PCA is an immunotherapy targeting prostate cancer antigens and is currently in the preclinical development stage.
Infectious Diseases
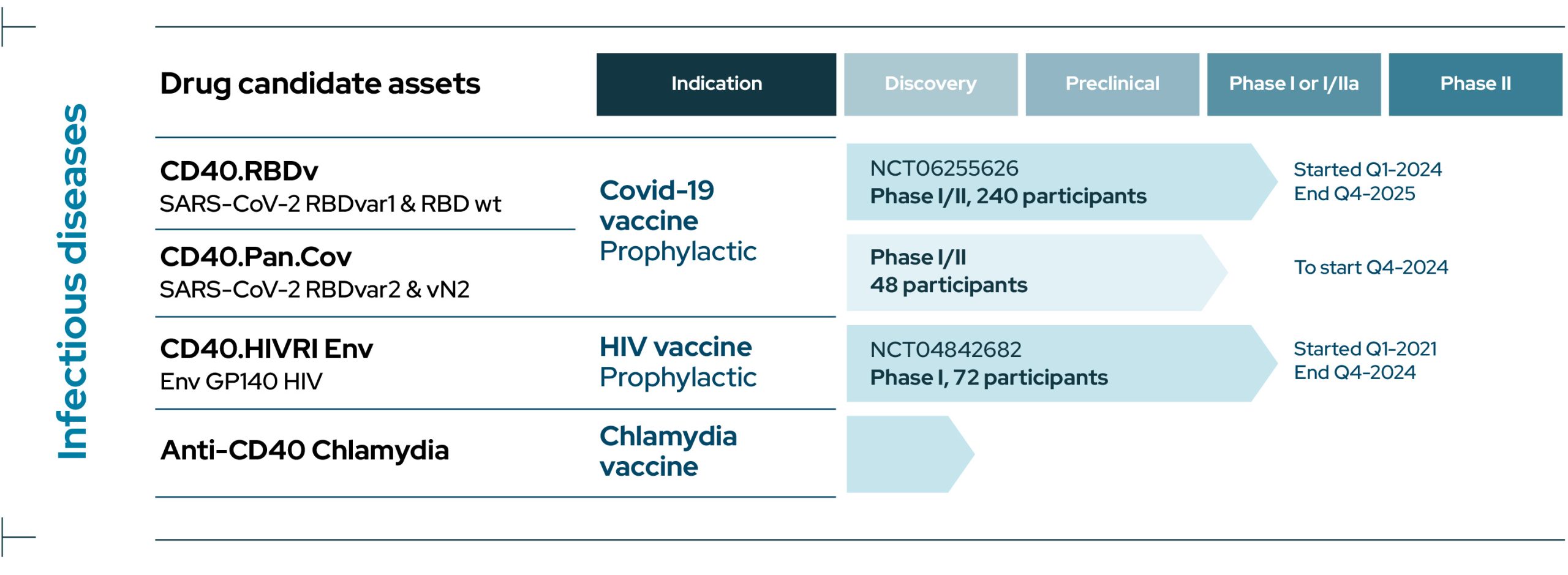
View Drug Candidate Assets
CD40.RBDv
CD40.RBDv is a prophylactic bivalent COVID-19 subunit protein vaccine containing both the ancestral SARS-CoV-2 sequence of RBD and the same portion of RBD harboring several mutations shared by several Variants of Concern (VOCs), including Omicron.
CD40.RBDv is currently evaluated in a Phase I/II clinical trial to test the safety and immunogenicity of the vaccine, adjuvanted or not, as a booster in volunteer (NCT06255626). This study is being conducted in collaboration with ANRS-MIE/Inserm.
CD40.Pan.Cov
CD40.Pan.Cov is a prophylactic COVID-19 vaccine aimed at boosting and extending pre-existing immune responses to antigens from various proteins of the SARS-CoV-2.
It is designed to target conserved regions from current SARS CoV-2 variants of concerns and Sarbecoviruses including RBD from spike harboring several mutations from VOCs and a highly conserved region from Nucleocapsid rich in T cell epitopes .
This vaccine is developed as a booster with a Phase I/II clinical trial due to start in Q4 2024 in collaboration with ANRS-MIE/Inserm.

CD40.HIVRI Env
CD40.HIVRI Env is a prophylactic vaccine targeting the Envelope of a clade C Human Immunodeficiency Virus (HIV).
In 2021-2024, a multicenter double-blind placebo-controlled Phase I/IIa dose-escalating trial was performed demonstrating safety and long-term immunogenicity of the vaccine in healthy volunteers in France and Switzerland (NCT04842682) in collaboration with ANRS-MIE/Inserm.
A second trial will start in 2025 in collaboration with the HVTN (HVTN318).
CD40.Chlamydia
We are conducting discovery research for a Chlamydia vaccine using EnnoDC CD40-vaccine technology.
Two candidate vaccines are under evaluation at the preclinical stage of development.
EnnoDC I Contact
If you wish to contribute, collaborate or learn more about EnnoDC, we would be delighted to hear from you.
Contact