Our groundbreaking and versatile antibody technology harnesses the power of immunotherapy and vaccines by stimulating dendritic cells.
Dendritic cells (DCs) are the most potent antigen-presenting cells in the immune system, to deliver a more targeted and effective immune response compared to current treatments. Activated DCs are crucial for the development of immune memory to identify and eliminate threats such as viruses and cancer.
I Versatile Antibody Technology
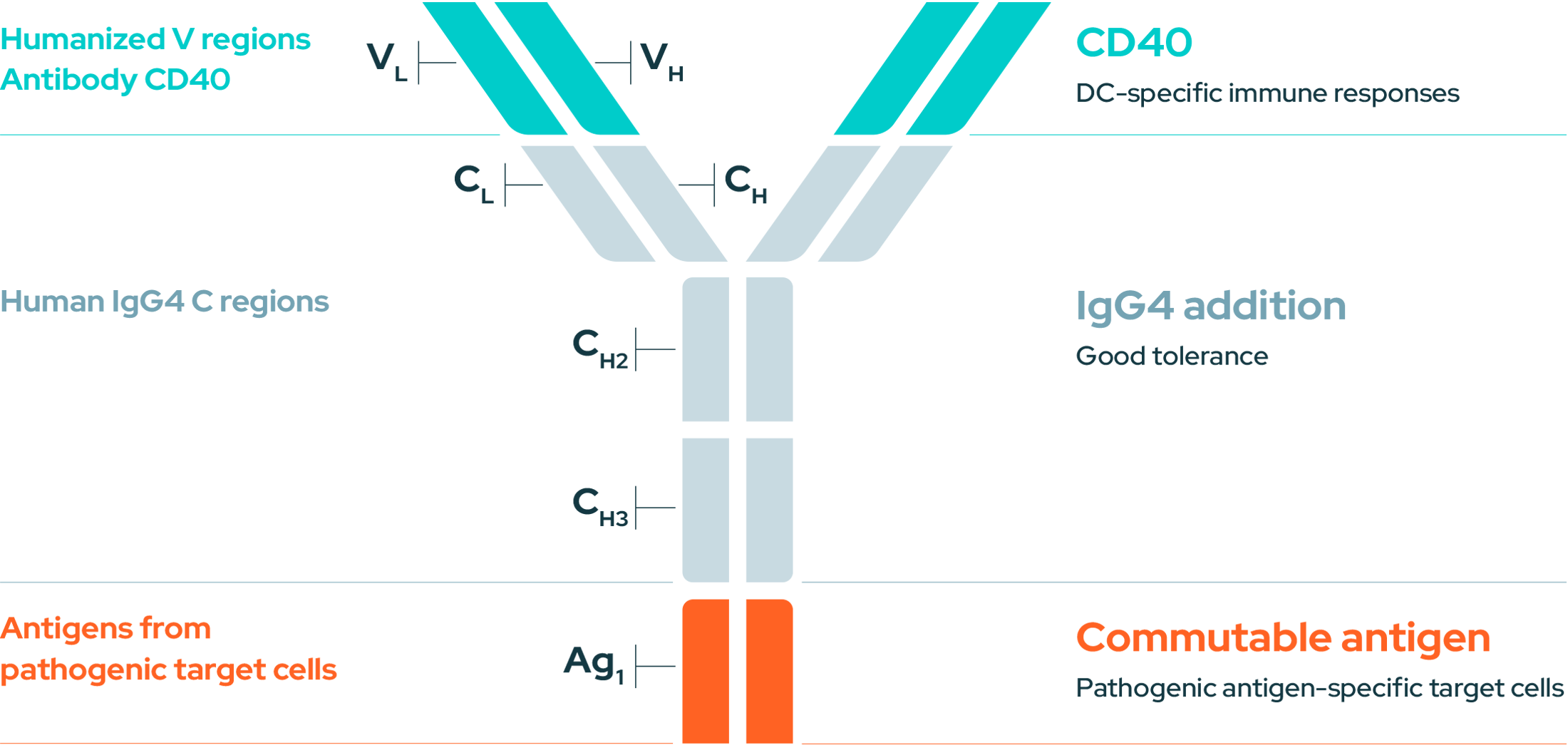
Our approach combines immunotherapy activation and vaccine specificity with a monoclonal antibody (mAb) that is fused with a commutable antigen and which targets DCs through CD40. The advantage of using these mAbs is that they provide a dual action approach, enhanced specificity and delivering antigens to DCs.
EnnoDC I Our Technology
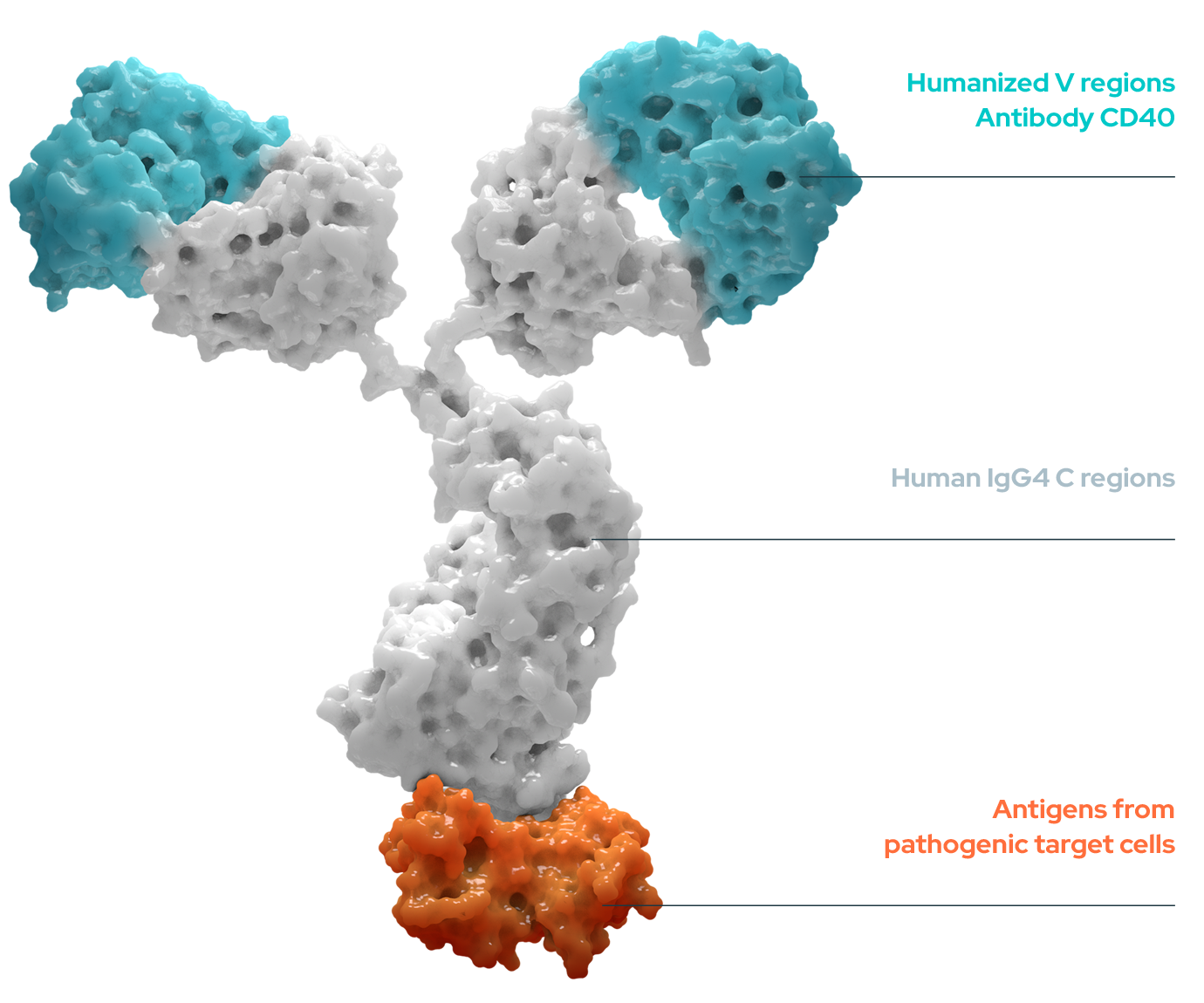

Anti-CD40
An immunotherapy combining mAbs and a commutable antigen targeting DCs through CD40 with the power of vaccine specificity.
I Immunotherapy activation
EnnoDC uses a novel, versatile, humanized anti-CD40 antibody. This antibody acts as a signal booster, stimulating DCs to educate and stimulate the immune system.
Dendritic cells are the most potent antigen-presenting cells in the immune system and activated DCs are crucial for the development of immune memory to identify and eliminate threats such as viruses and cancer.
I Vaccine specificity
EnnoDC’s anti-CD40 monoclonal antibody can be tailored to incorporate specific antigens from pathogens (viruses, bacteria) or cancer cells.
These antigens are delivered directly to the activated DCs, guiding the immune system to launch a targeted attack against the intended threat.
Education of the Immune System
Anti-CD40
Antibody + Antigen
EnnoDC anti-CD40 antibody is fused to specific antigen targeting DCs.
Target
Immature Dendritic Cell
The anti-CD40 with a commutable antigen targets an immature DC and is digested.
Activation
Mature Dendritic Cell
The DC digests anti-CD40, activates and presents fragments of the antigen.

Education
T Cell
DCs educate T cells to deliver a more targeted and effective immune response.
Effector Cells
T cells, cytotoxic T cells and B cells play a crucial roll in your immune system. While B cells send antibodies to kill harmful cells, cytotoxic T cells kill harmful cells directly.
CD8 T Cell
T Cell
T cells are a crucial part of identifying and killing infected cells.
CD4 T Cell
Helper T Cell
T cells are a crucial part of identifying and coordinating the immune response.
B Cell
Secreting Antibodies
B cells generate antibodies recognizing cancer cells and viruses.
Targets
We have a growing pipeline of novel immunotherapies, several of which are in clinical development. Target cancers include HPV16+ oropharyngeal and prostate cancer. Infectious disease targets include COVID and HIV.
EnnoDC I Platform
EnnoDC pioneers first-in-class DC-targeting CD40 and Ag-specific mAbs offering a significant improvement in safety and specificity as compared to classic CD40 mAbs.
I Below we show the comparison between the function of classic CD40 mAbs and the EnnoDC platform
Classic CD40 mAbs
Classic agonist CD40 mAbs induce increased CD40 signalling and toxicity
e.g. selicrelumab (Pfizer/Roche)
Classic agonist CD40 monoclonal antibodies (mAbs) interact with CD40 receptors on antigen presenting cells (APCs) which trigger downstream effects that lead to DC maturation and finally T cell priming to target cancer and infectious cells.
Due to CD40 expression on a broad range of cell types, CD40 activation through agonist CD40 mAbs can trigger severe adverse effects and toxicities such as cytokine storms, thrombocytopenia and liver toxicity.

Our Platform
CD40 12E12 IgG4 first-in-class ‘partial-agonist’ mAbs hamper toxicity-related mechanisms
12E12 is the founding clone from which EnnoDC candidates are developed
EnnoDC’s mAbs function through a partial-agonist mechanism that recognise CD40 receptors on the surface of DCs, inducing specific T cells, B cells and cytotoxic T cells responses against APCs while blocking CD40/CD40L interaction to prevent overstimulation and toxicity-related mechanisms.
EnnoDC I Our Approach
Advantages
01. Dual action
The EnnoDC first-in-class antibody combines the benefits of immunotherapy and vaccines activating the immune system while directing it towards a specific target.
02. Enhanced specificity
By delivering antigens directly to DCs, EnnoDC promotes a more focused immune response, minimizing potential side effects.
03. Promising future
EnnoDC holds immense potential for various applications, including battling cancer and fighting infectious diseases.
EnnoDC I Scientific Publications
Publications

EnnoDC is backed by a team of renowned scientists who regularly publish original research papers, the most relevant of which are accessible below.
Publications feature on websites outside of ennodc.com
View Publications
A vaccine targeting antigen-presenting cells through CD40 induces protective immunity against Nipah disease
Pastor Y, Reynard O, Iampietro M, Surenaud M, Picard F, El Jahrani N, et al.
Cell Reports Medicine
2024;5:101467.
Refining the DC-targeting vaccination for preventing emerging infectious diseases
Pastor Y, Ghazzaui N, Hammoudi A, Centlivre M, Cardinaud S, Levy Y.
Frontiers in Immunology
2022;13:949779.
Design, immunogenicity, and efficacy of a pan-sarbecovirus dendritic-cell targeting vaccine
Ceglia V, Zurawski S, Montes M, Kroll M, Bouteau A, Wang Z, et al.
Frontiers in Immunology
2022;12:786144.
Anti-CD40 Antibody Fused to CD40 Ligand Is a Superagonist Platform for Adjuvant Intrinsic DC-Targeting Vaccines
Ceglia V, Zurawski S, Montes M, Kroll M, Bouteau A, Wang Z, et al.
Frontiers in Immunology
2022;12:786144.
Targeting SARS-CoV-2 receptor-binding domain to cells expressing CD40 improves protection to infection in convalescent macaques.
Cheng L, Li G, Pellegry CM, Yasui F, Li F, Zurawski SM, et al.
Frontiers in Immunology
2021;12:672143.
TLR9- and CD40-Targeting Vaccination Promotes Human B Cell Maturation and IgG Induction via pDC-Dependent Mechanisms in Humanized Mice
Cheng L, Li G, Pellegry CM, Yasui F, Li F, Zurawski SM, et al.
Frontiers in Immunology
2021;12:672143.
Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10–producing suppressive CD4+ T cells
Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, et al.
The Journal of Experimental Medicine
2012;209(1):109-21
Anti-CD40 Antibodies Fused to CD40 Ligand Have Superagonist Properties
Ceglia V, Zurawski S, Montes M, Bouteau A, Wang Z, Ellis J, et al.
Journal of Immunology
2021.
TLR-9 agonist and CD40-targeting vaccination induces HIV-1 envelope-specific B cells with a diversified immunoglobulin repertoire in humanized mice
Godot V, Tcherakian C, Gil L, Cervera-Marzal I, Li G, Cheng L, et al.
PLoS Pathogens
2020;16(11):e1009025.
Gene Expression Signatures Associated With Immune and Virological Responses to Therapeutic Vaccination With Dendritic Cells in HIV-Infected Individuals
Thiebaut R, Hejblum BP, Hocini H, Bonnabau H, Skinner J, Montes M, et al.
Frontiers in Immunology
2019;10:874.
HIV-1 T cell epitopes targeted to Rhesus macaque CD40 and DCIR: A comparative study of prototype dendritic cell targeting therapeutic vaccine candidates
Flamar AL, Bonnabau H, Zurawski S, Lacabaratz C, Montes M, Richert L, et al.
PloS One
2018;13(11):e0207794.
TLR3 agonist and CD40-targeting vaccination induces immune responses and reduces HIV-1 reservoirs
Cheng L, Wang Q, Li G, Banga R, Ma J, Yu H, et al.
The Journal of Clinical Investigation
2018;128(10):4387-96.
Human innate responses and adjuvant activity of TLR ligands in vivo in mice reconstituted with a human immune system
Cheng L, Zhang Z, Li G, Li F, Wang L, Zhang L, et al.
The Lancet
2017;35(45):6143-53.
Superiority in Rhesus Macaques of Targeting HIV-1 Env gp140 to CD40 versus LOX-1 in Combination with Replication-Competent NYVAC-KC for Induction of Env-Specific Antibody and T Cell Responses
Zurawski G, Shen X, Zurawski S, Tomaras GD, Montefiori DC, Roederer M, et al.
Journal of Virology
2017;91(9).
Targeting HIV-1 Env gp140 to LOX-1 Elicits Immune Responses in Rhesus Macaques
Zurawski G, Zurawski S, Flamar AL, Richert L, Wagner R, Tomaras GD, et al.
PloS One
2016;11(4):e0153484.
Functional Specialty of CD40 and Dendritic Cell Surface Lectins for Exogenous Antigen Presentation to CD8(+) and CD4(+) T Cells
Yin W, Gorvel L, Zurawski S, Li D, Ni L, Duluc D, et al.
The Lancet
2016;5:46-58.
Yin W, Duluc D, Joo H, Xue Y, Gu C, Wang Z, et al.
Cancer Immunology Research
2016;4(10):823-34.
Therapeutic HPV Cancer Vaccine Targeted to CD40 Elicits Effective CD8+ T-cell Immunity
Graham JP, Authie P, Yu CI, Zurawski SM, Li XH, Marches F, et al.
Vaccine
2016;34(41):4857-65.
Targeting dendritic cells in humanized mice receiving adoptive T cells via monoclonal antibodies fused to Flu epitopes
Flamar AL, Contreras V, Zurawski S, Montes M, Dereuddre-Bosquet N, Martinon F, et al.
PloS One
2015;10(9):e0135513.
Delivering HIV Gagp24 to DCIR Induces Strong Antibody Responses In Vivo
Levy Y, Thiebaut R, Montes M, Lacabaratz C, Sloan L, King B, et al.
European Journal of Immunology
2014;44(9):2802-10.
Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load
Joo H, Li D, Dullaers M, Kim TW, Duluc D, Upchurch K, et al.
Immunity
2014;41(4):592-604.
C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses
Flamar AL, Xue Y, Zurawski SM, Montes M, King B, Sloan L, et al.
Aids
2013;27(13):2041-51.
Targeting concatenated HIV antigens to human CD40 expands a broad repertoire of multifunctional CD4+ and CD8+ T cells
Flamar AL, Zurawski S, Scholz F, Gayet I, Ni L, Li XH, et al.